RAS Pathway

RAS Pathway: Examining the Most Frequently Mutated Oncogene in Human Cancers
Almost a third of all cancers in patients are associated with mutations of the RAS family of genes, which includes KRAS, NRAS, and HRAS. There are other oncogenes, including EGFR and BRAF, that also activate the RAS pathway—meaning an even higher percentage of cancers depend on this pathway for growth and survival. Patients with a RAS-mutated cancer tend to experience worse outcomes and a higher disease burden than those without RAS pathway mutations. Watch the video to see how customized combinations of avutometinib, a unique RAF/MEK Clamp, including a combination with defactinib, a selective FAK inhibitor, have the potential to greatly expand the number of effective treatments for cancer patients who have limited options today.
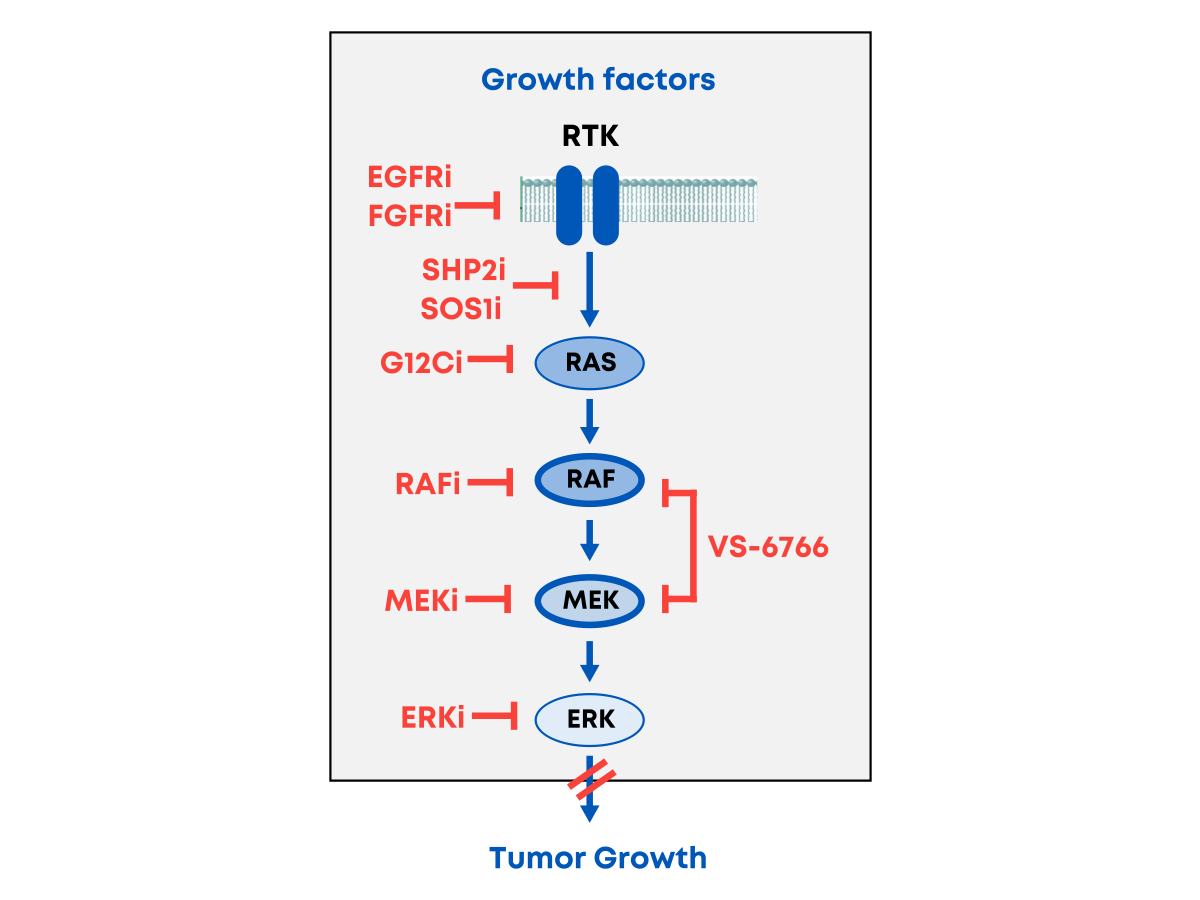
Blocking the RAS Pathway Presents Challenges Due to Multiple Resistance Mechanisms
Cancer has a strong affinity for the RAS pathway and reacts to the blocking of any single target by either reactivating the RAS pathway elsewhere or activating parallel pathways to survive. For example, MEK inhibitors paradoxically induce MEK phosphorylation (pMEK) and RAS signaling by relieving ERK-dependent feedback inhibition of RAF. MEK inhibitors also cause compensatory activation of phosphorylated FAK (pFAK), and BRAF inhibition also induces compensatory activation of pFAK. Single-target therapies (eg, MEK inhibitors) are associated with resistance and may not be the best avenue to slowing tumor growth—and finding tolerable combination regimens with MEK inhibitors has been challenging. There has been only modest progress and a limited number of approved therapies. Novel therapies and combinations are urgently needed to deliver on the promise of better outcomes for patients.
Avutometinib (RAF/MEK Clamp)
Defactinib (FAK Inhibitor)
Avutometinib as a Backbone of Therapy
Combining Avutometinib with Other Agents Gives It the Potential to Become a Backbone of Therapy
Strong preclinical synergy data have been demonstrated with vertical blockers, such as inhibitors of KRAS G12C, EGFR, SHP2, SOS1, and ERK1/2, and parallel pathway inhibitors of FAK, CDK4/6, and PI3K/AKT/mTOR, suggesting a broad range of possible combination partners. Avutometinib also offers favorable tolerability with an established twice-weekly dosing regimen, which may help patients stay on therapy longer.
By better controlling the RAS signaling network, customized RAS-targeted treatment combinations with avutometinib have the potential to greatly expand the number of effective treatment options for specific subsets of cancer patients who have limited options today. This can be life-changing.
Access additional information about Avutometinib treatment combinations